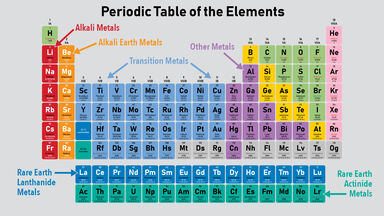
The periodic table on the left separates elements into three groups. The Elements of Metal.

Alkali Metal Definition Properties Facts Britannica
The types of metals on the Periodic Table can be further broken down by other properties but these are the basic types of metals.
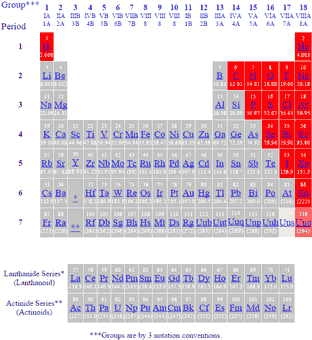
Which elements on the periodic table are metals. Also know are periodic tables mostly metals. Sodium fires are not put off with water because sodium reacts instantly with water and hydrogen gas evolves which burns with a pop sound by the effect of the heat of the reaction. In the periodic table the transition metals are present in eight groups 4 to 11 with some authors including some elements in groups 3 or 12.
The elements in these two rows are also referred to as respectively the lanthanide metals and the actinide metals. Elements of the group 1A in the periodic table are called alkali metals alkaline metals because they react with water forming alkaline solutions. Also many periodic tables have a stair-step line on the table identifying the element groups.
Metals are found on the left hand side of the table. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides. Of those the two most rare metals are rhodium Rh and osmium Os.
The elements in group 3 have an ns 2 n 1d 1 configuration. The line begins at boron B and extends down to polonium Po. The metalloids separate the metals and nonmetals on a periodic table.
They are grouped together in the middle to the left-hand side of the periodic table. Elements in group 1 and group 2 are metals. Although separate on the periodic table lanthanides and actinides are really specific types of transition metals.
Atomic structure and the periodic table. Like lanthanides they are radioactive are silver and soft. These metals are considered to be both very rare and of high value.
If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po. The elements are arranged in order of increasing atomic number with the lightest element hydrogen. This periodic table of the elements with names atomic number symbol and mass is color-coded for easier reference by students and researchers.
Chemical elements alphabetically listed The elements of the periodic table sorted by name in an alphabetical list. Some sections of the periodic table have special names. H in the top left hand corner.
On the periodic table there is a family of eight elements known as the precious metals including elements 44 47 like silver and 76 79 like gold. Continue exploring the Periodic Table by checking out element examples from all the types of elements. The metals are one of the three groups of elements as distinguished by their ionization and bonding properties along with the metalloids and nonmetals.
Most elements are metals. Metals are on the left of the periodic table and non-metals are on the right. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right.
Tend to be lustrous ductile malleable and good conductors of electricity while nonmetals are generally brittle if solid lack lustre and are insulators. An elements position on the Periodic Table tells us whether it is a metal a non-metal or a semi-metal. They are usually shiny very dense and only melt at high temperatures.
Metals reside on the left side of the table while non-metals reside on the right. This list contains the 118 elements of chemistry. In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84.
Actinides are also excluded from the main body of the periodic table but are also transition metals. The metals green in the table nonmetals orange and metalloids blue. Organizing the elements to help further our understanding was first provided by Dmitri Mendeleev.
Most elements can be considered metals. The two rows beneath the main body of the periodic table contain the inner transition metals. Click on any elements name for further chemical properties environmental data or health effects.
Atoms of group 1 elements have. Most elements are metals. This group includes alkali metals alkaline earth metals transition metals basic metals lanthanides rare earth elements and actinides.
The metals share several common properties including. Special Names for Sections of the Periodic Table. So because most elements of the Table are metals it makes sense to begin by looking at them.
Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. Elements of the periodic table are grouped as metals metalloids or semimetals and nonmetals. Metals In The Periodic Table.
Each element has a fixed position on the Periodic Table.

Layers Of Learning Science Hands On Experiments Family Style Learning Science Elementary Chemistry Chemistry
Classification Of Elements In The Periodic Table Color Coding Yellow Download Scientific Diagram