In the periodic table you can see a stair-stepped line starting at Boron B atomic number 5 and going all the way down to Polonium Po atomic number 84. Interactive periodic table with up-to-date element property data collected from authoritative sources.
The Periodic Table Chemistry Socratic
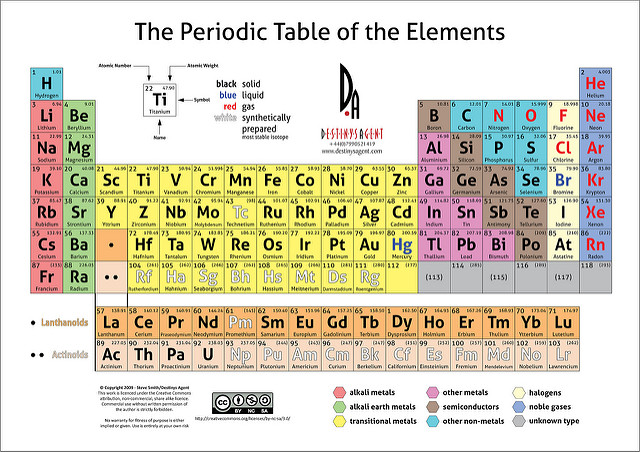
The periodic table also known as the periodic table of elements is organized so scientists can quickly discern the properties of individual elements such as their mass electron number electron configuration and their unique chemical properties.
Where are metals on the periodic table. Metals In The Periodic Table So because most elements of the Table are metals it makes sense to begin by looking at them. The elements with properties intermediate between those of metals and nonmetals are called semimetals or metalloids. Most elements can be considered metals.
The line begins at boron B and extends down to polonium Po. Interactive periodic table showing names electrons and oxidation states. The metals consist of the alkali metals alkaline earths transition metals lanthanides and actinides.
The first column in the periodic table are the Alkali Metals. The periodic table showing. Every element in this family has one valence electron that they will lose in order to achieve a pseudo-noble gas configuration.
Metals reside on the left side of the table while non-metals reside on the right. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. Look up chemical element names symbols atomic masses and other properties visualize trends or even test your elements knowledge by playing a periodic table game.
The periodic table is the tabular arrangement of all the chemical elements on the basis of their respective atomic numbers. The Periodic table can be divided into nine families of elements each having similar properties. The metals share several common properties including.
Alkali metals are named such because they react with water to form alkaline or basic solutions. Except for Germanium Ge and Antimony Sb all the elements to the left of that line can be classified as metals. When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
Elements to the left of the line are considered metals. The six alkaline earth metals are. The exception is hydrogen H the first element on the periodic table.
Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. This Periodic Table shows that there is nine different families some example are the Non metals and Alkali Metals and so forth down the line as seen on the image to the side. The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley which states that the properties of.
They are generally harder and denser than alkali metals have 2 electrons in their outermost s sub-shell and each make a distinct color in their flames. The elements can be placed in the periodic table. They are grouped together in the middle to the left-hand side of the periodic table.
Metals in most of the left and centre metalloids in a narrow diagonal band nonmetals in the right plus hydrogen. If you look at the Periodic table you will find that the metal elements are located between atomic number 5 Boron B all the way to atomic number 84 Polonium Po. Elements to the far right of the periodic table are nonmetals.
As shown in Figure 28 Types of Elements metals occupy the left three-fourths of the periodic table while nonmetals except for hydrogen are clustered in the upper right-hand corner of the periodic table. Get essential facts about the first 20 elements all in one convenient place including the name atomic number atomic mass element symbol group and electron configurationIf you need detailed facts about these elements or any of the higher numbered ones start with the clickable periodic table. The position of an element provides information about its properties.
The alkaline earth metals are found in column 2 on the left side of the Periodic Table. Visualize trends 3D orbitals isotopes and mix compounds. Periodic table of the elements materials science and academic information elements and advanced materials data scientific presentations and all pages designs concepts logos and color schemes herein are the copyrighted proprietary rights and intellectual property of American Elements.
Elements just to the right of the line exhibit properties of both metals and nonmetals and are termed metalloids or semimetals. Most elements are metals with different properties to those of non-metals. At ordinary temperatures and pressures hydrogen behaves as a nonmetal.
In the periodic table the vertical columns are called groups and the horizontal rows are called periods.

Metals And Non Metals Of The Periodic Table

The Parts Of The Periodic Table
How Can You Differentiate Between Metals And Nonmetals On The Periodic Table Socratic

Heavy Metal Position In Periodic Table Download Scientific Diagram
