The non metalsin periodic table are mainly in the upper right position The only chemical element that is not found in this region of the table is Hydrogen which is located in the upper left corner together with Alkaline Metals but since it behaves in most circumstances as a Non-Metal it is classified as such. Non-metals are characterized by having the exact opposite properties of metals.
The nonmetal elements occupy the upper right-hand corner of the periodic table.
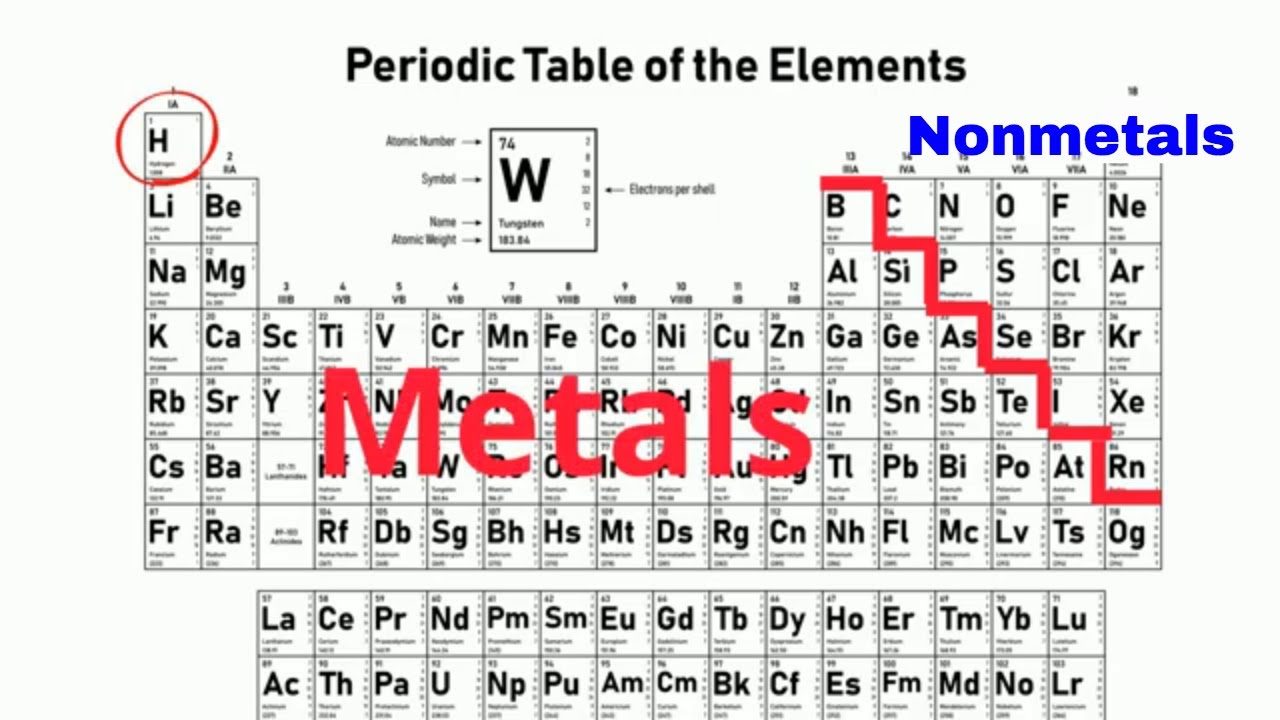
Where are the nonmetals on the periodic table. The nonmetals list which makes up the periodic table includes hydrogen helium carbon sulfur nitrogen oxygen radon neon other halogens and noble gases etc. Non-Metals In The Periodic Table. The reactive nonmetals near the metalloids show some incipient metallic character such as the metallic appearance of graphite black phosphorus selenium and iodine.
The nonmetals in the periodic table. They are separated by a diagonal band of semimetals. The only exception to this is atomic number 1 Hydrogen H which has a different location on the table.
Elements that are nonmetals are hydrogen carbon nitrogen phosphorus oxygen sulfur selenium all of the halogens and the noble gases. These elements have similar chemical properties that differ from the elements considered metals. Nonmetals are located on the right side of the periodic table.
Non-metals can be easily located on the Periodic Table because they are to the right of the line that looks like a stepping ladder. Atoms to the left of the. Well you have got the answer of Where are Nonmetals located on the Periodic Table.
Non metals in periodic table. The nonmetals are a group of elements in the periodic table. The nonmetals are brittle not malleable or ductile poor conductors of both heat and electricity and tend to gain electrons in chemical reactions.
Nonmetals react by gaining electrons rather than losing electrons because they have a lot of electrons on the outermost shell making is easier to gain a few electrons. Hydrogen is a nonmetal which is located on the left top corner of the Periodic table. This line is often referred to as the staircase because of its shape.
On most versions of the Periodic Table hydrogen is placed with the metals even though it has physical properties similar to those of the non-metals it is a gas at room temperature. Most of the elements that make up the human body are nonmetals. Nonmetals are located on the upper right side of the Periodic table see above image.
Nonmetals on the Periodic Table. Colour all the blocks to the right of the semi-metals red. Non-metal elements are on the right of the stepped line Metals are on the left of the periodic table and non-metals are on the right.
Nonmetals have properties opposite those of the metals. Now you can also colour hydrogen H red. Nonmetals are located on the far right side of the periodic table except hydrogen which is located in the top left corner.
ADVERTISEMENT When we study the elements it is important to know which elements are metals and which ones are not. Nonmetals are separated from metals by a line that cuts diagonally through the region of the periodic table containing elements with partially filled p orbitals. The nonmetal element group is a subset of these elements.
Metals are lustrous good conductors of electricity and readily shaped they are ductile and malleable whereas solid nonmetals are generally brittle and poor electrical conductors. Metals Nonmetals and Metalloids on the Periodic Table - YouTube A description and practice of finding metals nonmetals and metalloids on the Periodic TableIn general metals are found on the. The noble gases are almost completely inert.
The nonmetals are located on the upper right side of the periodic table. Nonmetals include the nonmetal group the halogens and the noble gases. They are located to the right of the metalloids and to the left of the halogens.
These elements are often referred to as other nonmetals as the halogens and noble gases are also nonmetals. Polyatomic nonmetals have structures with either three nearest neighbours as is the case for example with carbon in its standard state of graphite or two nearest neighbours for example in the case of sulfur. Nonmetals with the exception of hydrogen are located on the right side of the periodic table.
The only nonmetal that is a liquid at room temperature is _____. The halogens and noble gases are nonmetals but the nonmetal element group usually consists of the following elements. All these elements are non-metals.
Metals are located on the left of the periodic table and nonmetals are located on the upper right. These elements are shown in the following figure. From left to right in the periodic table the nonmetals can be divided into the reactive nonmetals and the noble gases.
Some nonmetals are liquids. The nonmetals are in the last few columns of the periodic table because they have from 4-8 electrons is the outermost shell putting them groups 13-18 on the periodic table. Atomic structure and the periodic table Elements in group 1.
Most periodic tables print a thick black line to show the division between metals and nonmetals. Moving rightward across the standard form of the periodic table nonmetals adopt structures that have progressively fewer nearest neighbours.

The Periodic Table Is Made Up Of Metals Nonmetals And Metalloids Periodic Table Of The Elements Periodic Table Geometry Worksheets



