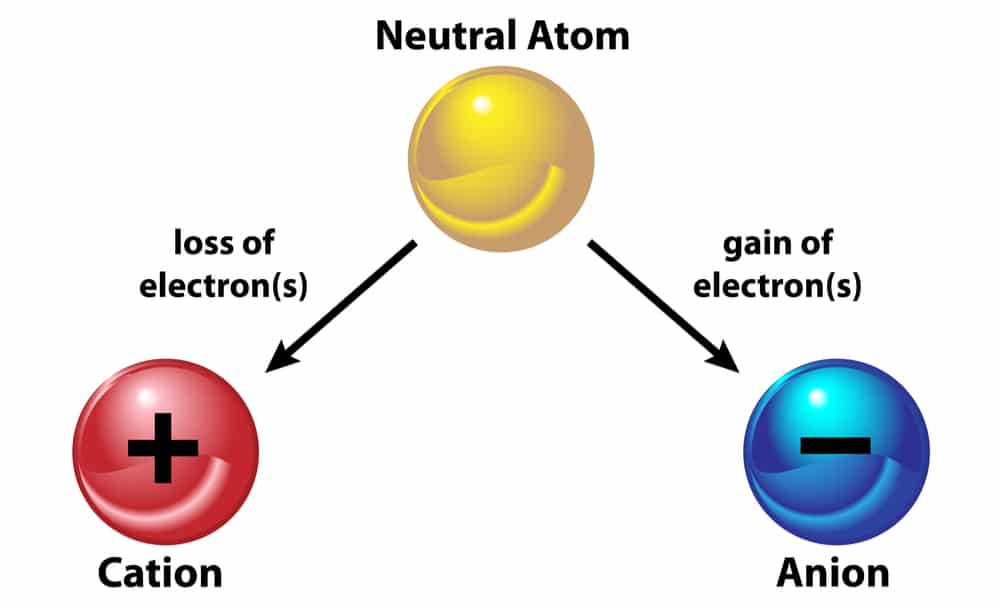
If the atom loses one or more electrons it becomes a positively-charged ion if the atom gains one or more electrons it becomes a. They lose electrons and are oxidised.
/illustration-of-electron-transfer-from-sodium-atom-to-chlorine-atom-transformation-from-sodium-ion-96168913-58a5a8c13df78c345be8cc31.jpg)
What Is An Ion Definition And Examples
Ions that carry a positive charge are called a anions b cations c mineralytes d Course Hero Ions that carry a positive charge are called a anions 34.
Ions that carry a positive charge are called. Ions are charged particles. If an atom or atoms lose electrons or gain protons the ion has a positive charge. Ions that carry a positive charge.
It is called a cation. The sodium ion shown above is formed from the loss of one. Positive Ions lose electrons Negative Ions name.
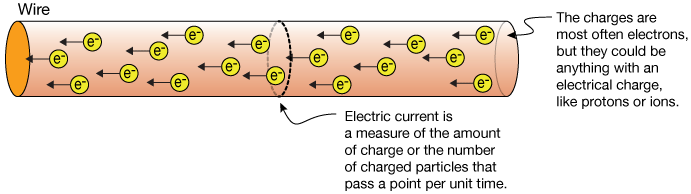
Cations positively-charged ions and anions negatively-charged ions are formed when a metal loses electrons and a nonmetal gains those electrons. The electrostatic attraction between the positives and negatives brings the particles together and creates an ionic compound such as sodium chloride. Ions electrically charged atoms can carry a positive or negative charge.
Ions that carry a positive charge are called which of the following terms. The progression goes like this. Metal atoms lose electrons to form positively.
An ion with a positive charge is called a cation and has a superscript sign to the right of it an ion with a negative charge is called an anion and has a superscript sign to the right of it. Discovered the nucleus and the concentrated positive charge in the nucleus. Ion with a positive charge.
And ions that have a positive charge are called cations. Ions that carry a positive charge are called a. Also known as ferroxidase I.
But if the atom loses some electrons it will have more positive charges than negative charges and is called a positive ion. Negatively charged ions move to the positive electrode during electrolysis. Ions can be grouped into two broad categories.
The progression goes like this. They are often involved in static electricity and electric current on electrolyte solutions such as salt water. A copper-dependent enzyme that enables iron to bind to transferrin.
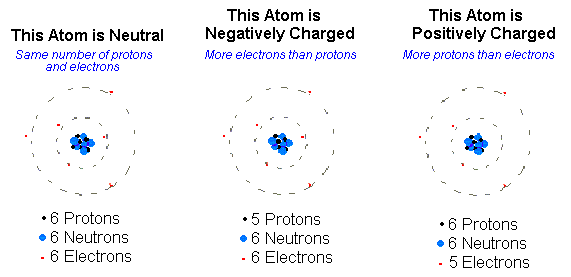
Elements are made of tiny particles called atoms 2 All atoms of a given element are identical. Ions form when atoms lose or gain electrons to obtain a full outer shell. An ion has a positive charge cation or a negative charge anion.
Ions that carry a positive charge are called. Likewise if the atom gains an excess of electrons it is called a negative ion. Negatively charged ions are anions formed from nonmetallic elements like oxygen and sulfur.
Ions are formed by the addition of electrons to or the removal of electrons from neutral atoms or molecules or other ions. And ions that have a positive charge are called cations. AskedOct 24 2015in Nutritional Scienceby Wendy.
In electrolytes such as salt water the charge carriers are ions which are atoms or molecules that have gained or lost electrons so they are electrically charged. Enjoy our search engine Clutch Save a GPA. An ion with a positive charge is called a cation and has a superscript sign to the right of it an ion with a negative charge is called an anion and has a superscript sign to the right of it.
Atoms that have gained electrons so they are negatively charged are called anions atoms that have lost electrons so they are positively charged are called cations. Ions that carry a negative charge. Negatively charged ions are anions formed from nonmetallic elements like oxygen and sulfur.
Wavelike motion of small hairlike projections on some cells. Atom or atoms gain electrons or lose protons the ion will have a negative charge. Positively charged ions are called cations.
Name changes with ending of ide ex. Cations are ions that carry a net positive charge because the number of protons in the species is greater than the number of electrons. And ions that have a positive charge are called cations.
Or by rupture of a covalent bond between two atoms in such a way that both of the electrons of the bond are left in association with one of the formerly bonded atoms. By combination of ions with other particles. When they do they form charged particles called ions.
Ion with a positive charge. Ions electrically charged atoms can carry a positive or negative charge. An ion is an atom or group of atoms with a positive or negative charge.
A molecule hasnt an electrical charge. The sodium ion shown above is formed from the loss of one. Ions that carry a positive charge are called which of the following terms.
Negatively charged ions anions. The substance that is broken down is called the electrolyte. And ions that have a positive charge are called cations.
We made it much easier for you to find exactly what youre looking for on Sciemce. The formula for a cation is indicated by a superscript following the formula that indicates the number of the charge and a sign. Donate your notes with us.
The Anion Cation Connection In Soil Ecofarming Daily

Ions Charged Atoms Ppt Download