Naming Basic Ionic Compounds 1. Lets say the ionic compound youre working with is NaCl.
5 7 Naming Ionic Compounds Chemistry Libretexts
The anion is named by taking the elemental name removing the ending and adding -ide.
How are ionic compounds named. Some anions have multiple forms and are named accordingly with the use of roman numerals in parenthes. NaCl Sodium Chloride. You first write the name of the metal lithium and then write the name of the nonmetal adding an -ide ending so that sulfur becomes sulfide.
The naming of ionic compounds is dependent upon the type of ionic molecule formed from alkali metals alkaline earth metals or transition metals. If the first element you see in the compound is found on the left side of the periodic table ie. An ionic compound is formed by the complete transfer of electrons from a metal to a nonmetal and the resulting ions have achieved an octet.
Suppose that you want to name the compound that results from the reaction of lithium and sulfur. The most common type of ionic bonding is seen in compounds of metals and. Consult the periodic table of elements.
An atom of element D has 20 electrons. Ternary compounds are composed of three or more elements. How to Name Ionic and Covalent Compounds.
Practice naming ionic. When naming ionic compounds all of the information youll need is on the. In ionic compounds a cation is the positive part of the compound that loses electrons and the anion is the negative part of the compound that gains electrons.
The cation has the same name as its element. Ethe symbol for the metal in each of the compounds in Model 2. For example K 2 O is called potassium oxide.
Left of the metalloid staircase it is a metal and the compound is ionic When naming ionic compounds we never indicate the ratio of component elements in the name - that is for covalent compounds only. A chemical compound is a chemical substance composed of many identical molecules composed of atoms from more than one element held together by chemical bonds. But when melted in an aqueous solution they act as strong electrolytes and conduct electricity.
Binary Metal to Non-Metal When a metal and non-metal form an ionic molecule the metal will retain the element name and the non-metal will taken the suffix -ide. The resulting compound is called an ionic compound and is said to be held together by ionic bonding. Naming Ionic Compounds Using -ide H - Hydride F - Fluoride O 2- Oxide S 2- Sulfide N 3- Nitride P 3- Phosphide.
The first part of the name is the name of the metal element. When you name binary ionic compounds with transition metals the rules are the same as those of binary ionic compounds. When you name ionic compounds you write the name of the metal first and then the nonmetal.
How are they different from ionic compounds. Working out a formula The formula for an ionic. The name of the metal is written first followed by the name of the nonmetal with its ending changed to ide.
For example sodium chloride melts at 801 C and boils at 1413 C. Ionic compounds are compounds made up of ions. Which element comes first in the name and formula of the compounds in Model 2the metal or the nonmetal.
The simplest chemical formulas are for binary compounds - compounds made up of two elements. Positive and negative charges must balance. Ions of two different elements where a metal element and it combines with a nonmetal element.
Ionic compounds are named by stating the cation first followed by the anion. An ionic compound is named first by its cation and then by its anion. How Ionic Compounds Are Named.
View Ionic_Naming from CHEMISTRY 000 at University High Orlando. N aCl Sodium Chloride. The table shows the names and formulae of some common ions.
In this tutorial we will be learning how to name ions and ionic compounds from the formula and how to find the formula from the compound name. Jot down the formula of the ionic compound. Binary ionic compounds typically consist of a metal and a nonmetal.
So that the charges are balanced. For a two element ionic compound the naming is simple. For example K 1 is called the potassium ion just as K is called the potassium atom.
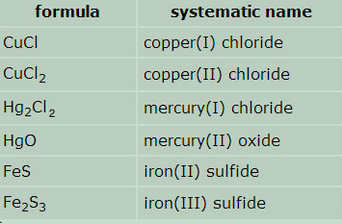
Odcl 2 Ionic Compound Names Metals that form one ion Sodium chloride Calcium sulfide S Silver sulfide n P Zinc phosphide Aluminum oxide el Strontium chloride 4. The anion is named by taking the elemental name removing the ending and adding ide. The cation has the same name as its element.
Write the name of the metal. For example K 1 is called the potassium ion just as K is called the potassium atom. A what are binary ionic compounds.
The second part is the name of the nonmetal element with the suffix -ide Here are some examples. To deduce the formulae of ionic compounds the formulae of their ions can be used. The first part.
An ionic compound is named first by its cation and then by its anion. This lesson will demonstrate how to write a chemical formula for a binary ionic compound when given the systemic name. In ionic compounds there arise characteristic distances between ion neighbours from which the spatial extension and the ionic radius of individual ions may be derived.
Ionic and molecular compounds are named using somewhat-different methods.

How Are Ionic Compounds Named What Do The Numerical Prefixes Used In Naming Covalent Compounds Tell You What Does A Compound S Empirical Formula Ppt Download
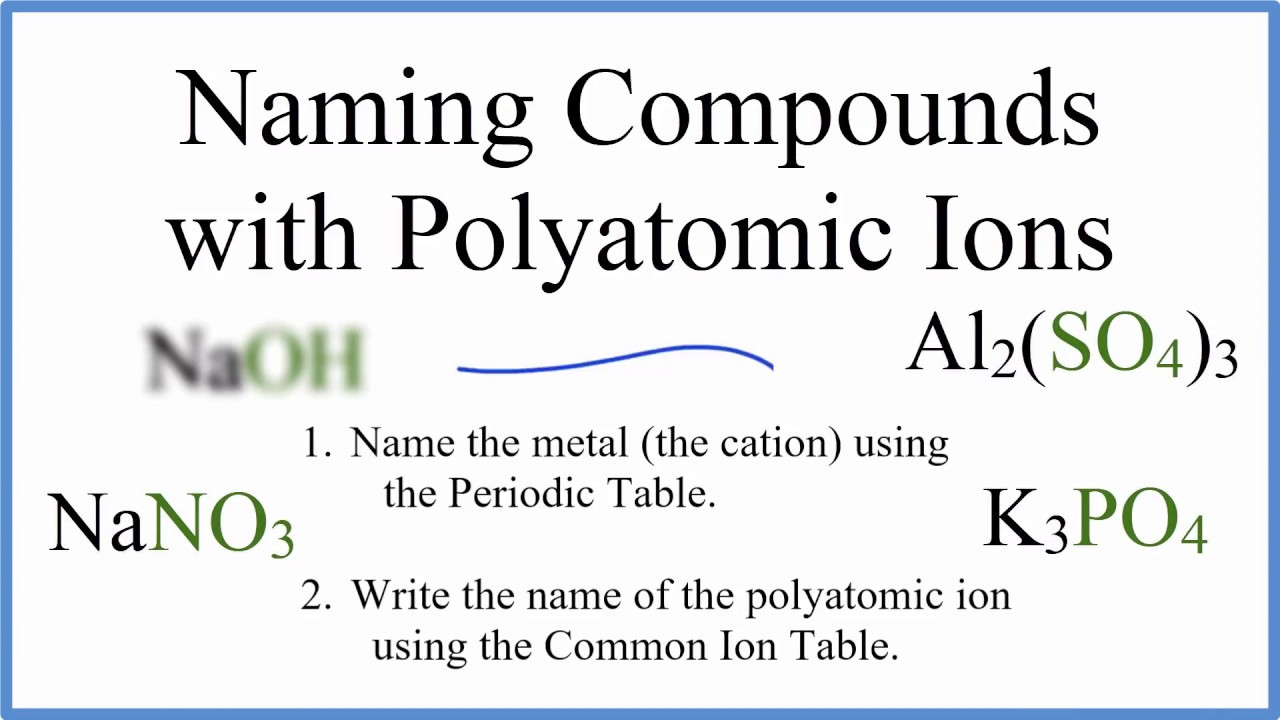
How To Name Ionic Compounds With Polyatomic Ions Youtube

Naming Ions And Ionic Compounds Video Khan Academy
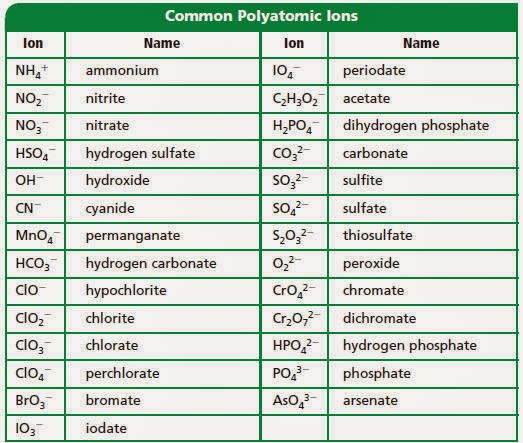
Naming Type I And Ii Polyatomic Ionic Compounds Ms J Kim S Science Classes
