When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties. The halogen family consists of fluorine chlorine bromine iodine and astatine.

On The Periodic Table What Is A Family Familyscopes
Families of the periodic table.
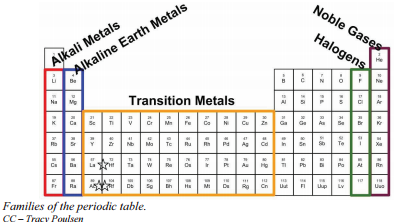
What are families on the periodic table. The columns of the periodic table are often used to define families. Halogens form ionic bonds with all kinds of elements. In addition to the blocks listed in this table there is a hypothetical g-block which is not pictured here.
They all have seven valence electrons which make them highly reactive for covalentbonds. Element Families on the periodic table is a set of elements sharing common properties. Group 8A of the periodic table.
The number of elements in a period increases as you move down because there are more sub levels per level as the energy level of the atom increases. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. Note that this element family includes nonmetals semimetals and metals.
There are 18 numbered groups in the periodic table. Alkali metals alkaline earth metals halogens noble gases transition metals etc. The periods represents the energy level of an atom.
Families and Periods In the periodic table of elements there are seven horizontal rows of elements called periods. In chemistry a group also known as a family is a column of elements in the periodic table of the chemical elements. The elements in a group have similar physical or chemical characteristics of the outermost electron shells of their.
That column is labeled Group Zero. Alkali Metals Alkaline Earth Metals BoronAluminum Group Icosagens Carbon Group Crystallogens Nitrogen Group Pnictogens Oxygen Group Chalcogens Halogens and Noble Gases. Elements in each family.
Members of the families vertical columns in the periodic table have similar properties. The vertical columns of elements are called groups or families. Families of the periodic table DRAFT.
The center of the periodic table contains the transition metals plus the two rows below the body of the table lanthanides and actinides are special transition metals. Alkali Metals Group 1 of the periodic table are the alkali metals. They are highly reactive and do not occur freely in nature.
The noble gases are all located in the far right column of the table. Families on the Periodic Table. The largest family of elements consists of transition metals.
Most element families are a single column of the periodic table although the transition elements consist of several columns plus the elements located below the main body of the table. The table is made up of rows known as periods and columns known as groups or families. Other families can be made of elements in a series.
G-block elements can be seen in the expanded extended periodic table. Elements on the periodic table can be grouped into families bases on their chemical properties. The Periodic table can be divided into nine families of elements each having similar properties.
The elements are classified into families because the three main categories of elements metals non metals and semi- metals are very broad. The f-block columns between groups 2 and 3 are not numbered. Many chemists prefer and still use this method.
The families are labeled at the top of the columns in one of two ways. An example of an element family is the nitrogen group or pnictogens. To differentiate it from the other families in the periodic table.
This product includes eight mini - posters one for each of the element families on the main group of the periodic table. Each family has a. Which color on the image of the periodic table corresponds with the alkaline earth metals.
A vertical column in the periodic table. There are 7 periods in the Periodic Table. The most common way the periodic table is classified by metals nonmetals and metalloids.
Periodic table Helium is placed next to hydrogen instead of on top of neon because it is part of the s2 group. The older method uses Roman numerals and letters. Groups 3 to 12 of the periodic table.
The newer method uses the numbers 1 through 18. Are located in the second column from the right side of the periodic table group 17. Periods are the horizontal rows of elements found in the Periodic Table of Elements.
Group 1A of the periodic table. Group 7A of the periodic table. Group 2A of the periodic table.
The lanthanide and actinide series below the body of the periodic table are transition metals too. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical propertiesThe structure of the table shows periodic trendsThe seven rows of the table called periods generally have metals on the left and nonmetals on the right. Periodic Table Families The periodic table of elements is a way of organising the vast array of different elements found in chemistry.
Properties Of Chemical Families On The Periodic Table By Gabrielle Juarez

Periodic Table Element Families Diagram Quizlet

Color And Learn About The Periodic Table Layers Of Learning Periodic Table Of The Elements Teaching Science Physical Science Middle School
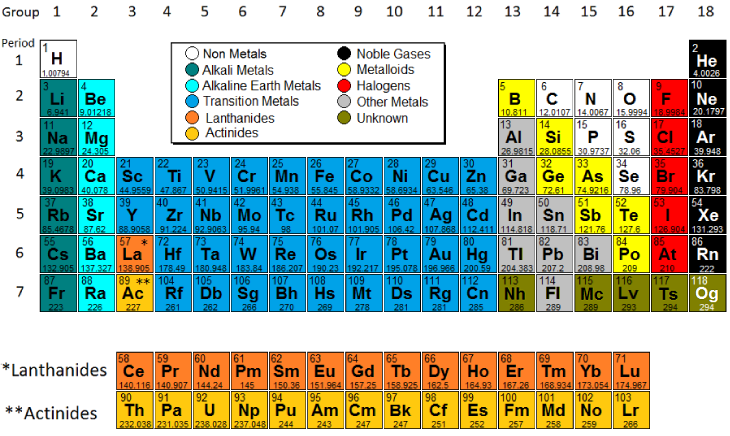
/periodic-table-165930186-590f2d703df78c92832fe141.jpg)