Elements in the same group have similar chemical properties. Periodic Table of Elements - American Chemical Society.

In The Periodic Table How Many Columns And Groups Are There Quora
Members of the families vertical columns in the periodic table have similar properties.
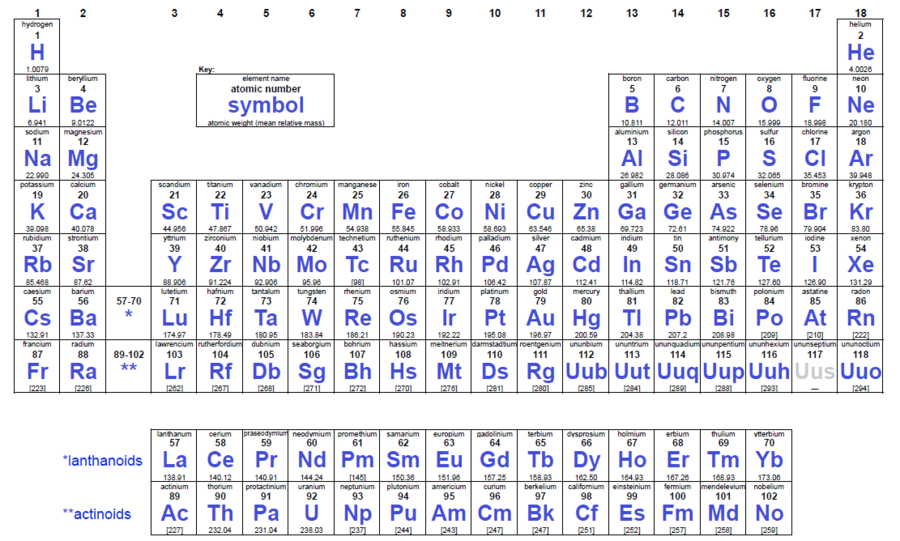
Periodic table vertical columns. The most common way the periodic table is classified by metals nonmetals and metalloids. The periodic table also known as the periodic table of elements is a tabular display of the chemical elements which are arranged by atomic number electron configuration and recurring chemical properties. Chemistry Periodic Table Stock Illustrations 12464.
The vertical columns of the Periodic table are called groups. Physics 21062019 1640 masonsee4ytube. Arranged this way groups of elements in the same column have similar chemical and physical properties reflecting the periodic lawFor example the halogens lie in the second-last.
Periods in the periodic table. The Same group elements have similar properties and reactivity. The vertical columns of elements are called groups or families.
The groups of the periodic table are displayed as vertical columns numbered from 1 to 18. The table he produced had elements with similar properties fall into the same vertical column. The elements in any one vertical column are in the same group.
There are 18 groups on the periodic table and elements that are members of the same group share similar traits. The vertical columns in the periodic table are called. Each Vertical Column Of The Periodic Table Is Called A.
For example the number of valence electrons in the group 1 is 1. In the periodic table of elements there are seven horizontal rows of elements called periods. Sendus your suggestions so we can make it better.
A period in the periodic table is a row of chemical elementsAll elements in a row have the same number of electron shellsEach next element in a period has one more proton and is less metallic than its predecessor. In 1869 a Russian chemist named Dmitri Mendeléev published a periodic table. Of elements were related to their atomic mass in a repeating or periodic way and arranged them so that groups of elements with similar properties fell into vertical columns in his table.
The structure of the table shows periodic trends. The column of an element tells us more about its properties because elements in the same column share most of their properties with the other elements from the same group which can help us deduce different things about them. 1 Get Other questions on the subject.
Groups are the vertical columns in the periodic table. The groups are numbered from left to right. The elements in a group have very similar chemical properties which arise from the number of valence electrons presentthat is the number of electrons in the outermost shell of an atom.
Significance of the names and symbols of the elements. These are the horizontal rows that show the number of shells of electrons an atom has. Most of the times the elements in the same group shares somewhat similar chemical and physical properties.
He arranged the elements known at the time in order of increasing relative atomic mass and showed that elements of similar properties reoccurred at regular intervals. Vertical columns on the periodic table are called _____ The elements in each column have_____ For 1 For 2 Agrouping ASimilar element names Bfamilies BSimilar properties Cperiods CSimilar symbols Dlines DSimilar masses was asked on May 31 2017. Each group of elements having the same number of valence electrons.
Each element within the periodic table has its own block. Vertical Columns On The Periodic Table Are Called. The vertical columns on the period table are called groups.
The elements of the periodic table are arranged by increasing atomic number. The elements of a given column have similar properties. The s- p- and d-block elements of the periodic table are arranged into these columns or groups.
Because of this members of the same groups tend to receive and release the same number of electrons which means elements in the same group share reactivity traits. What is the force acting on the object in the horizontal direction. The seven rows of the table called periods generally have metals on the left and nonmetals on the right.
The columns called groups. Find an answer to your question What do the columns on a periodic table of elements represent in English if youre in doubt about the correctness of the answers or theres no answer then try to use the smart search and find answers to the similar questions. All the chemical elements in the same group contain an equal number of valence electrons.
All elements in the same group have the same number of electrons in their outer electronic shell. In each period horizontal row the atomic numbers increase from left to right. Facts About the Elements on the First.
Elements are arranged on the Periodic Table in order of increasing atomic number where each element has one proton more than the element preceding it. Columns in the periodic table groups The vertical columns in the periodic table are called groups. These elements are then wrapped into rows and columns corresponding to the properties of the elements in each row and column.
Aforce of 345 newtons is applied to an object at an angle of 45 º with the horizontal. The 18 vertical columns of the table are called Groups. The table is arranged in vertical columns called Groups numbered 1 8 and in rows called Periods.
The number of electrons in the.

Periodic Table Of Elements Knowino
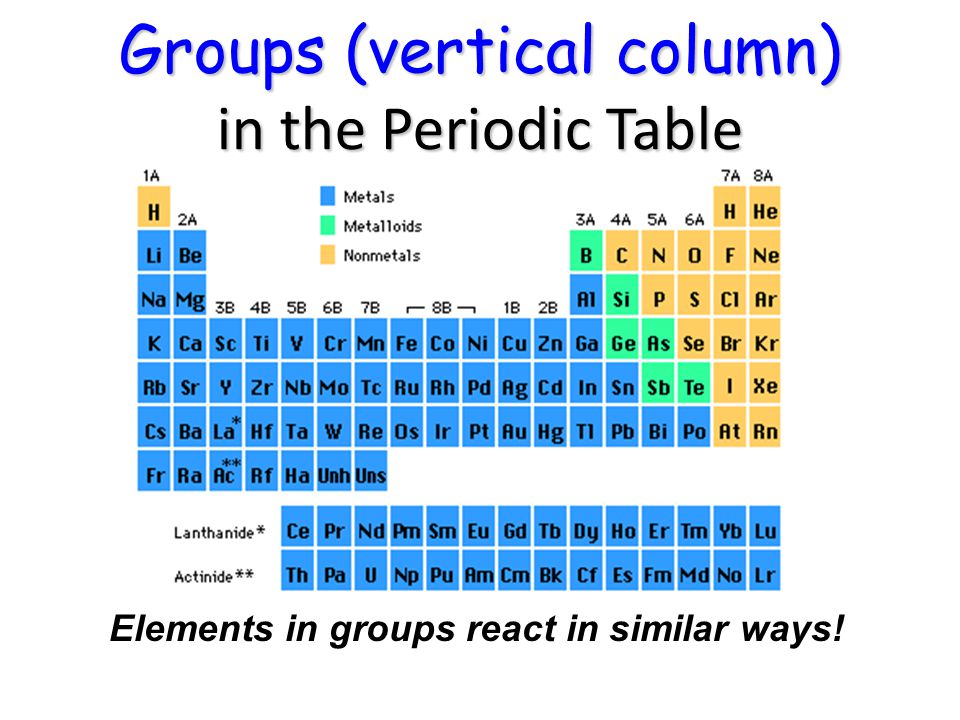
Periodic Table Ppt Video Online Download
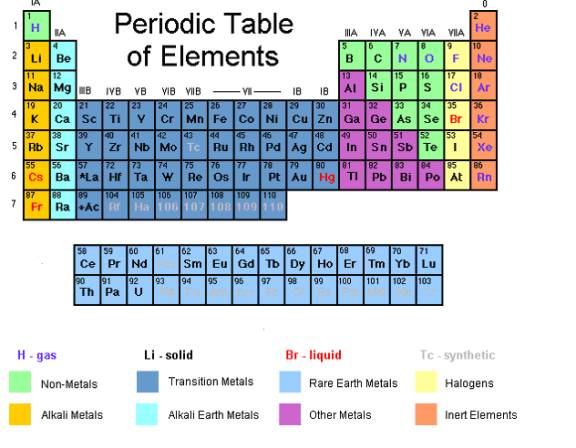
Periodic Table Of Elements With Worked Solutions Videos

Modern Periodic Table The Modern Periodic Table Consists Of 7 Horizontal Periods And 16 Vertical Groups Periodic Table Chemistry Periodic Table Online Science